Decisions, decisions, decisions
written by Maree Stuart
Being in a lab isn’t always easy, especially when there are decisions to be made. According to prospect theory, if two equal options are put to a person but one option is described in relation to gains and the other is described according to losses, the person is more likely to choose the first option. It makes sense- we all like to feel like we’ve won.
One decision making area where labs often get stuck is with the requirements on decision rules. Let’s look at what is actually required and how you can make these decisions easy.
What’s a decision rule?
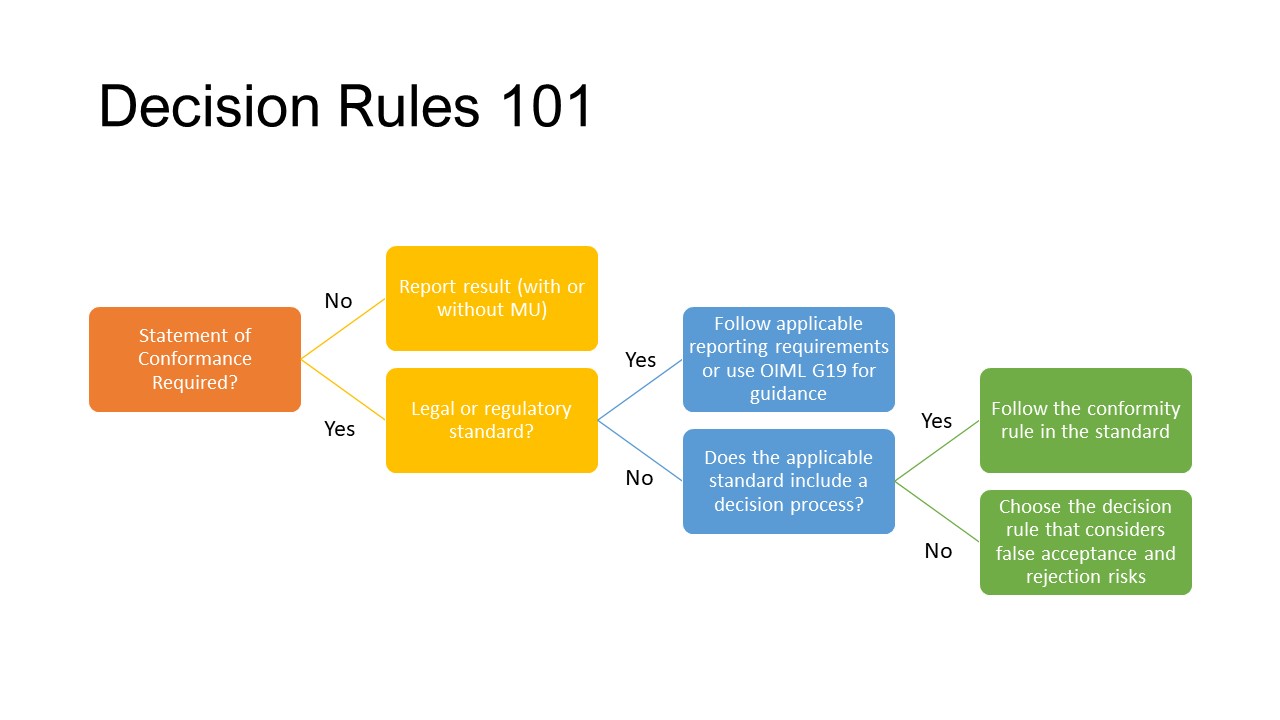
A decision rule is a rule that describes how measurement uncertainty is accounted for when stating conformity with a specified requirement. Oh dear- that thorny topic of MU!
Every time we make a decision, we inherently do a risk calculation. A decision rule is a risk transfer mechanism. If a lab is making a decision about whether a product meets a specification, or where an analyte conforms with a regulatory requirement, they bear the risk as to the consequences of that decision, especially if they get this wrong.
What the application of a decision rule does is to transfer the risk from the lab to the true decision maker, the client or regulator.
The before and after of decision rules
ISO/IEC 17025 has two kinds of requirements when it comes to decision rules.
It starts way back when a lab accepts work from a client. The first decision to be made is a fairly simple one – is the client asking you to make a statement of conformity to a specification, standard or regulation?
If the answer is “No”, then go no further. If you’re not sure or it’s a “Yes”, then you do need to engage with the customer.
 I know that this is where things can get stuck. Lots of lab customers do not actually know what specifications or standards are relevant to their request. They appoint the lab as the “God of decisions” and just know that they have to get some testing or calibration done in a NATA lab. Some careful questions about why the testing or calibration is to be done can uncover this kind of information. It requires a more nuanced customer intake process.
I know that this is where things can get stuck. Lots of lab customers do not actually know what specifications or standards are relevant to their request. They appoint the lab as the “God of decisions” and just know that they have to get some testing or calibration done in a NATA lab. Some careful questions about why the testing or calibration is to be done can uncover this kind of information. It requires a more nuanced customer intake process.
But….. nobody has time for this in-depth conversation with the client, right? Labs are busy places and as many people have said, life would be so much simpler if there were no clients.
Whatever the customer intake process you establish, you need to communicate the decision rule to the customer and get their agreement up front.
One way to manage this is to have some clauses in the terms and conditions that customers sign up to when they submit their request. How would you like to handle the decisions? Options include:
- Just report the result and its uncertainty and make no statement of conformance
- Report the result as a pass or fail, but only if there is a clear margin between the result and its uncertainty and the specification limits or regulatory threshold
- Report the result as a conditional pass or fail with a note that the conformance is not clear when the MU of the result is applied.
Of course, all options present different levels of risk for a lab. What would be the consequences if a client accepted your approach as stated in terms and conditions and they suffered a loss? To help labs with this, there’s this little extra piece of information in clause 7.8.6.1 of ISO/IEC 17025 that this decision rule must take into account the level of risk (such as false accept and false reject and statistical assumptions) associated with the decision rule employed. What on Earth does that mean?
It means when the decision rule is not already inherently a part of a standard other people have to think about the risk, especially the consequences, if the call of conformity is incorrect.
There are a few guidance documents on the topic including ILAC’s G8 Guidelines on Decision Rules and Statements of Conformity and OIML G19 The role of measurement uncertainty in conformity assessment decisions in legal metrology. These can help with setting up the decision rule with a consideration of how risk plays into this.
So it’s all systems go. We have agreement on the decision rule to be employed and it’s the “right” decision rule! Now what?
After the testing or calibration work is complete and it’s time to report, then you need to implement the decision rule your client has agreed to. That means, you need to know the specification or limit, the result and the MU. That’s a lot of information to have on hand when all you want to do is send out the report! Are your lab systems up to this? If not, then perhaps that’s an area for improvement.
If you do have this information at hand the report also needs to include the decision rule employed. If you routinely report results against specifications or regulatory limits. It’s a good idea to have this rule as a standard statement in your report templates.
If reporting statements of conformity is a little less common, think about the reporting processes you’ll need to employ. Automation systems can help.
Do you need help with making these kinds of decisions and improving some aspects of your systems? Get in touch- we have expertise across all of these areas! Call Maree on 0411 540 709, or email info@masmanagementsystems.com.au for a chat about the options.
Remember, you don’t have to do this alone!
Download the article Decisions decisions decisions

